PRINCE
PRINCE ("Preclinical Information Center") is Bayer’s one-stop search assistant and platform for finding preclinical research data, developed in collaboration with Thoughtworks. The platform consolidates over 17,000 study reports and their metadata into a searchable, cloud-based system built on AWS. By making decades of scientific data and records easily accessible, PRINCE acts as a assistive co-pilot to help researchers design more efficient studies, speed up repetitive tasks, and accelerate the decision-making process in preclinical research and pharmaceutical drug discovery.
Building on this foundation, a generative AI chatbot was integrated onto the platform, enabling scientists and project managers to search and query sensitive, internal data in natural language. Each response is grounded in source-cited information to ensure transparency and trust. Together, PRINCE and its AI assistant aims to transform how Bayer’s teams interact with research data, turning static reports into actionable insights that support innovation across safety, toxicology, and preclinical development research, and more.
PROBLEM
DESIGN PROCESS
(Some visual designs have been modified as per NDA requirements)
RESEARCH & DISCOVERY
User studies revealed common challenges and opportunities.
We kicked off the project with a series of user interviews on internal Bayer researchers who were already using an early release of the PRINCE chatbot. Users ranged from toxicologists, microbiologists, R&D innovation directors, and machine learning research scientists. The outcome of these user interviews were opportunities that helped to identify persona, user goals, potential use cases, and common challenges faced when interacting with PRINCE. Some questions explored included the following:
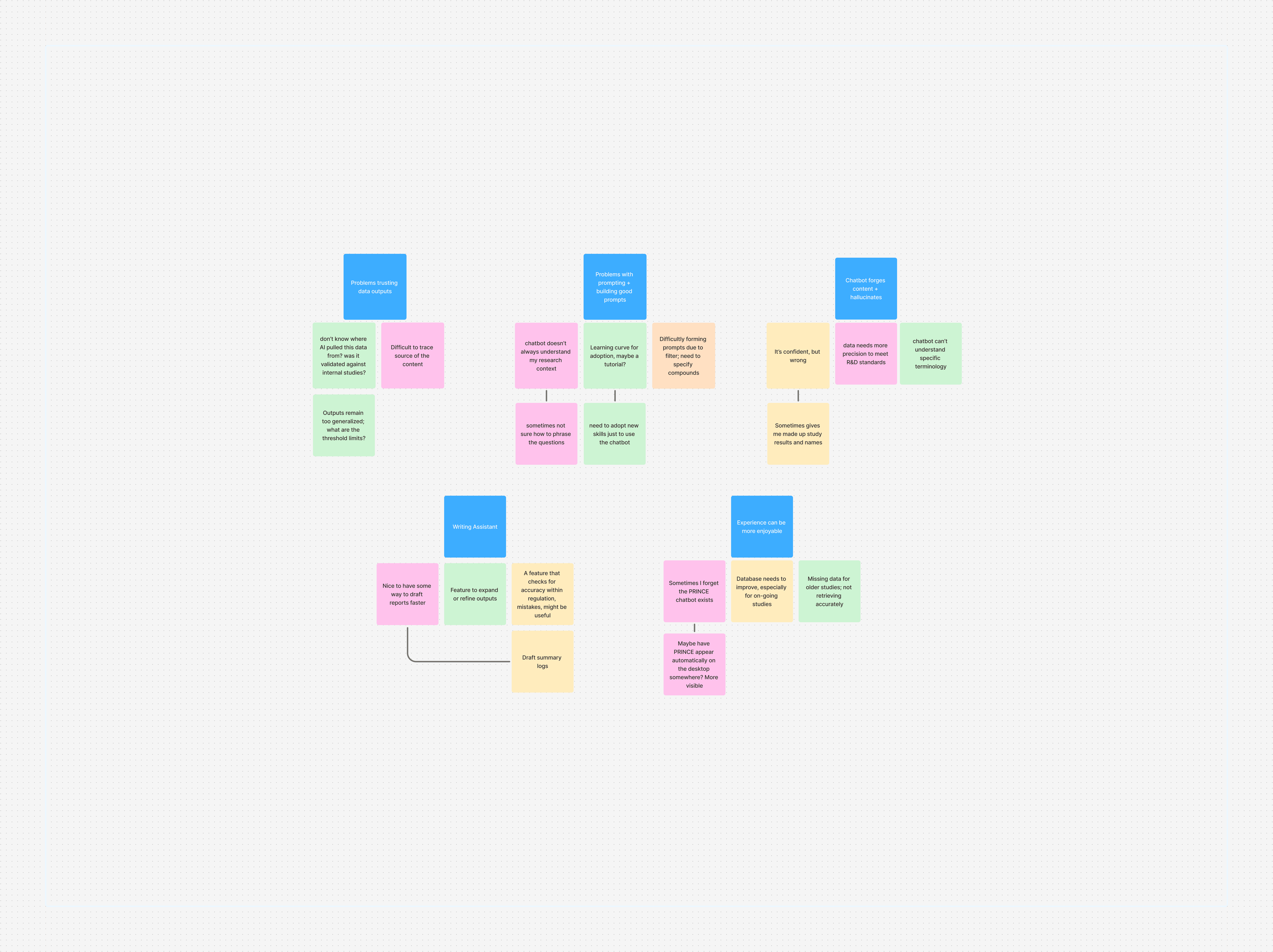
Affinity Mapping identified an actionable product roadmap.
After uncovering key insights into how our users interact and leverage AI systems in their workflows, I translated the user findings into a affinity map. The goal was to identify common challenges and actionable use cases for possible features engineers could implement into the next product sprint.
PROTOTYPES
CONVERSATION STARTERS
What if we add starter prompts and topic modes to help onboard new users into the interface?
Since we found that new users were unsure how to begin interacting with the AI chatbot, we introduced conversation starters, a predefined, context-aware example of prompts users can select to begin their search query and initially test out the system’s capabilities. The idea was to help improve user onboarding and user confidence, reducing hesitation when adopting a new tool. Another concept we explored was introducing topic modes, a predefined agent setting to perform and solve task-based problems.
TRACING SOURCES
How can we offer a way for users to access the sources for each search request within the internal database?
Researchers needed transparency to trust AI-generated responses and validate the origin of retrieved information. We visualized a sources section that lists and links back to the original study report from the internal database for each derived answer. This strengthened trust, accountability, and compliance by making each LLM generated response fully traceable to a verified data source.
DEPLOYMENT PREVIEWS
What about a real-time preview log showing each decision the chatbot deploys to reach its final output?
Researchers needed greater visibility into how the AI processed their queries, as the system’s reasoning and retrieval steps were largely opaque. Therefore, a deployment preview feature was added to surface the 'behind-the-scenes' process of the LLM—showing how inputs were interpreted, data retrieved, and outputs were formed. This transparency also created space for potential human-in-the-loop interventions, allowing specialists to review or refine steps at specific stages before final results were generated.
FINAL DESIGNS
We ended on a clean, simple, and familiar user interface.
The new PRINCE interface brought forward a cleaner, user-friendly interface with simplified features. The LLMs models were also fine tuned to receive better tokens during the information retreival process, since AI hallucinations were a major concern for researchers. Users reported a more positive experience navigating the PRINCE database, and provided constructive feedback on potential feature add-on's in future iterations.
OUTCOMES
Impact & Awards
The new PRINCE interface was shipped and demoed at an internal Bayer showcase attracting over 500+ virtual attendees. It presented a cleaner user interface, conversation starters to help onboarding, accurate logs of intermediate steps for better transparency, as well as an updated side bar menu for conversational history. It received positive remarks from users in our test group and was also awarded Bayer's GenAI Award for the best technical implementation in early Spring 2025.
Reflections & Learnings
Overall, this project taught me that designing for AI is not about showcasing intelligence, it's about designing for human behaviour, transparency, and trust. The final PRINCE prototype transformed from a basic search tool into a trusted research collaborator, built to promote transparency, security, and a fluid human-centered user experience. Throughout this process, I collaborated closely with AI engineers, gaining a hands-on understanding of tokenization, retrieval-augmented generation (RAG), and foundational large language model (LLM) concepts to better align my product decisions for what was possible within the system's capabilities.